Addressing PFAS in the Environment Part 2: Anchor QEA’s Bioaccumulation Model
As discussed in the first article in this blog series, per- and polyfluoroalkyl substances, or PFAS, are a complex family of synthetic chemicals found in soils, sediments, groundwater, surface water, and the atmosphere—as well as in aquatic and terrestrial organisms including people. The U.S. Environmental Protection Agency (EPA) has published health advisories for perfluorooctane sulfonic acid (PFOS) and perfluorooctanoic acid (PFOA), two of the most common PFAS.
There are over 5,000 PFAS, and our understanding of this family of chemicals is complicated by the fact that once released into the environment, many PFAS transform into other PFAS. This transformation chain eventually leads to one of several terminal end products called perfluoroalkyl acids, or PFAAs, such as PFOS and PFOA. Understanding the behavior of PFAA precursors in the environment, especially their transformation, is important when setting water quality criteria, establishing fish consumption advisories or regulations, and addressing contaminated sites.
Water Quality Criteria and Fish Consumption Advisories to Tackle PFOS Contamination
Drinking water criteria and fish consumption advisories for a subset of PFAS have been established recently in various states and jurisdictions. PFOS is of particular interest when evaluating risks due to consumption of aquatic organisms because it has been shown to bioaccumulate in fish, wildlife, and humans. Assessing PFOS exposure may be more complicated than assessing exposure to other chemicals, such as PCBs, because PFOS precursors and intermediate transformation products can be found in the environment and in organisms.
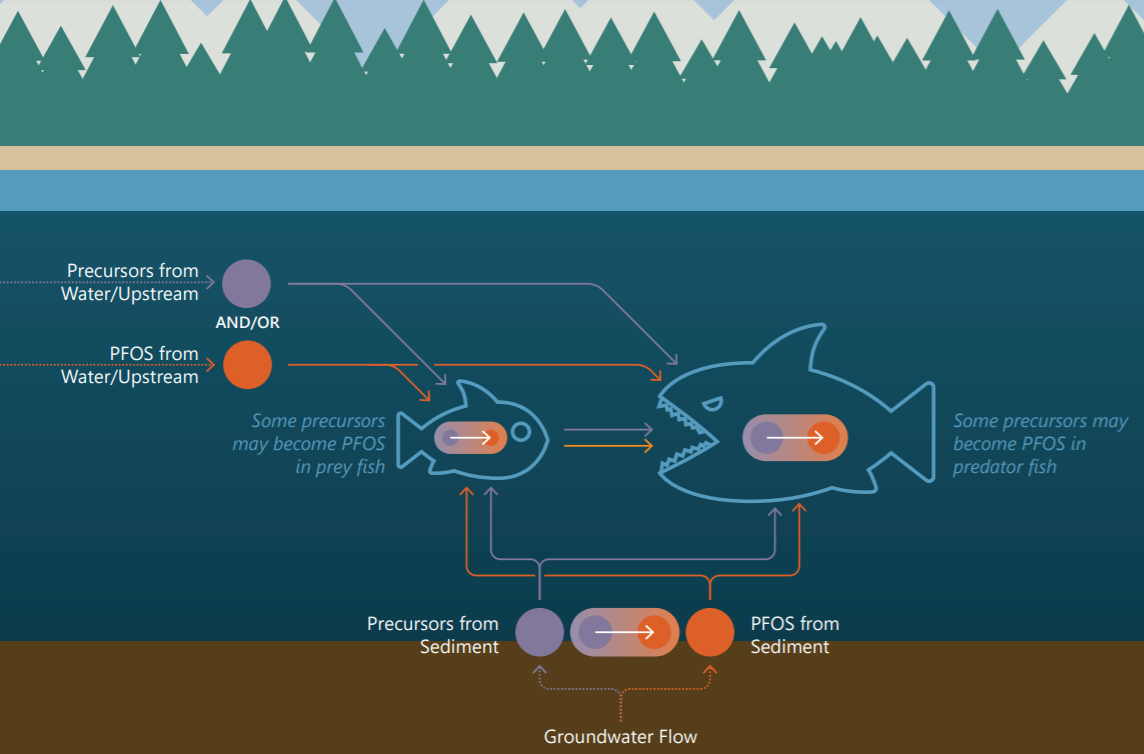
Prioritizing PFOS sources in aquatic food webs can be difficult due to the possibility of uptake of both PFOS and precursors from multiple sources, as well as precursor transformation in the sediment, water, and fish tissue.
The PFOS to which wildlife or people are exposed may have been released directly into the environment, may be the biotransformation product of discharged PFOS precursors, or both. Consequently, the development of remedial strategies may depend on the relative concentrations and properties not only of PFOS itself, but also of its precursors. This situation may make the source of PFOS difficult to identify and trace in the environment.
Bioaccumulation Model: Understanding Role of Precursors in Sediments
Bioaccumulation modeling is often used to investigate contaminant sources and to inform remedial actions designed to reduce exposure to contaminants. Incorporating transformation processes into bioaccumulation models is crucial to understanding precursors’ contributions to the PFOS concentrations found in fish. Anchor QEA has developed a model to help bridge the gap between risk assessment, PFOS bioaccumulation, and decision-making for contaminated sites.
Our bioenergetics-based model simulates the uptake and loss of PFOS and PFOS precursors in a cascade of transformations that terminate in PFOS, in a simple food web consisting of a predator fish and a prey fish. Specifically, the model represents SAmPAP, a PFOS precursor that was a major component in paper coatings produced in the United States and has been detected in sediment in significant concentrations. The model incorporates growth, respiration, and consumption rates. It was developed using data from peer-reviewed literature, including two laboratory experiments that incorporated fish exposure to SAmPAP in food and in water.
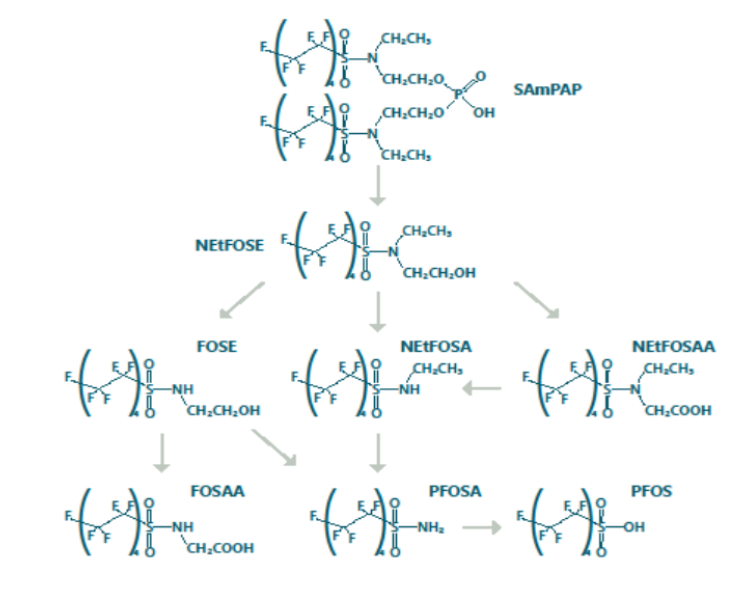
This model represents the biotransformation cascade from SAmPAP to PFOS in fish tissue. It includes water and sediment exposure and food web biomagnification.
Where precursors are present, PFOS concentrations in fish may be higher than expected based only on exposure to PFOS in water. This insight is critical to the development of water criteria based on fish tissue contamination. Our modeling shows that SAmPAP, at levels which have been measured in sediments can produce PFOS concentrations in fish tissue that approach fish advisory levels. And because SAmPAP strongly sorbs to sediment, it may be a long-term source of PFOS in fish. Therefore, reducing PFAA concentrations in fish requires some idea of the importance of precursors such as SAmPAP, as well as their sorption properties and transformation rates. Although it is not yet possible to identify all fluorinated compounds in the environment, some methods are being developed to estimate the total mass of fluorinated compounds (e.g., the Total Oxidizable Precursor Assay and Total Organic Fluorine). These methods may also provide some indication of the existence of PFAA precursors.
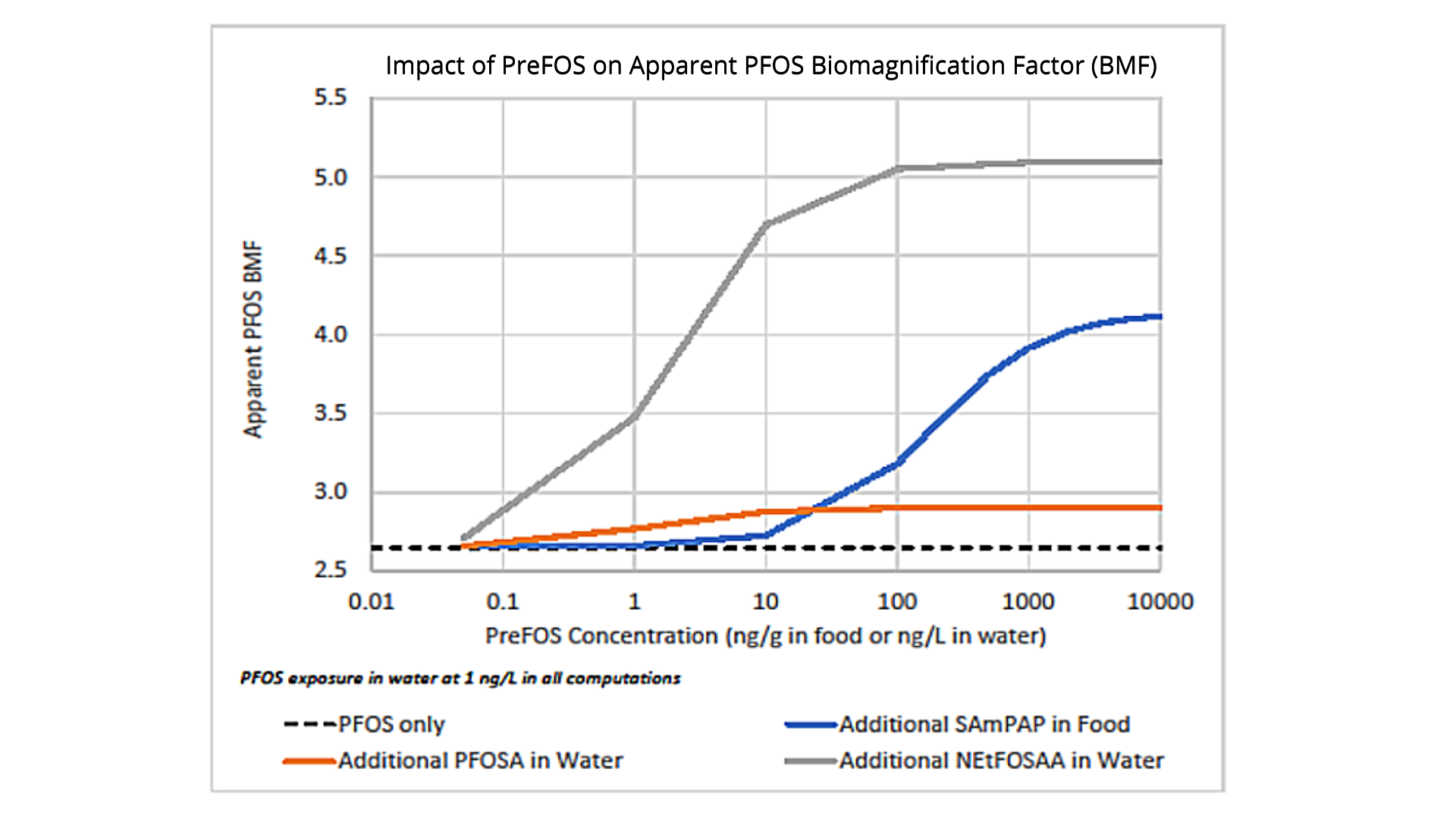
Model results showing the impact of precursors on the apparent PFOS biomagnification factor (BMF) in fish. In each simulation, fish were exposed to 1 ng PFOS/L plus an additional precursor, either in food or water. As precursor concentration increases, the apparent PFOS BMF increases.
Although precursors may constitute a long–term source of PFOS in fish, if the precursor source can be remediated, the rapid depuration of PFOS from fish will allow fish concentrations to recover quickly. Compared to the many years of recovery time anticipated with other chemicals (e.g., some PCBs), once the source of high PFOS concentrations—such as SAmPAP—is removed, the food web is anticipated to recover in a matter of months.
The goal of this modeling work is to support remedial decision-making by helping identify the sources of PFOS in aquatic organisms. The model is a tool that can be used to assess when precursors are at sufficiently high levels to meaningfully contribute to PFOS concentrations in fish. Ultimately, understanding precursors and their biotransformation will be key to successfully identifying PFOS sources and removing these chemicals from the environment. A full description of the model is available in the paper, “The Impact of Precursors on Aquatic Exposure Assessment for PFAS: Insights From Bioaccumulation Modeling,” available online in Integrated Environmental Assessment and Management (SETAC).

