Addressing PFAS in the Environment Part 3: Anchor QEA’s Sediment and Surface Water Model
August 26, 2021 – Elizabeth Lamoureux, Senior Managing Scientist, and David Glaser, PhD, Principal
Per- and polyfluoroalkyl substances (PFAS) are a complex family of synthetic chemicals found in soils, sediments, groundwater, surface water, and the atmosphere—and in aquatic and terrestrial organisms, including people. The more than 5,000 PFAS are drawing increasing regulatory interest, primarily because we are exposed to them in the water we drink and the fish we eat.
A subset of PFAS, perfluoroalkyl acids (PFAAs), account for most regulations and health concerns. In addition to the regulations established in various states and jurisdictions, the U.S. Environmental Protection Agency (EPA) has published health advisories for perfluorooctane sulfonic acid (PFOS) and perfluorooctanoic acid (PFOA), two of the most common PFAAs. EPA has also proposed to regulate PFAS in drinking water as a class and is considering whether to regulate PFOS and PFOA as hazardous substances.
The Fate and Transport of PFAAs: The Role of Precursors
Regulation and remediation of these chemicals are complicated by the fact that once released into the environment many PFAS transform into other PFAS. These transformation pathways eventually lead to the PFAAs, which are the terminal end products.
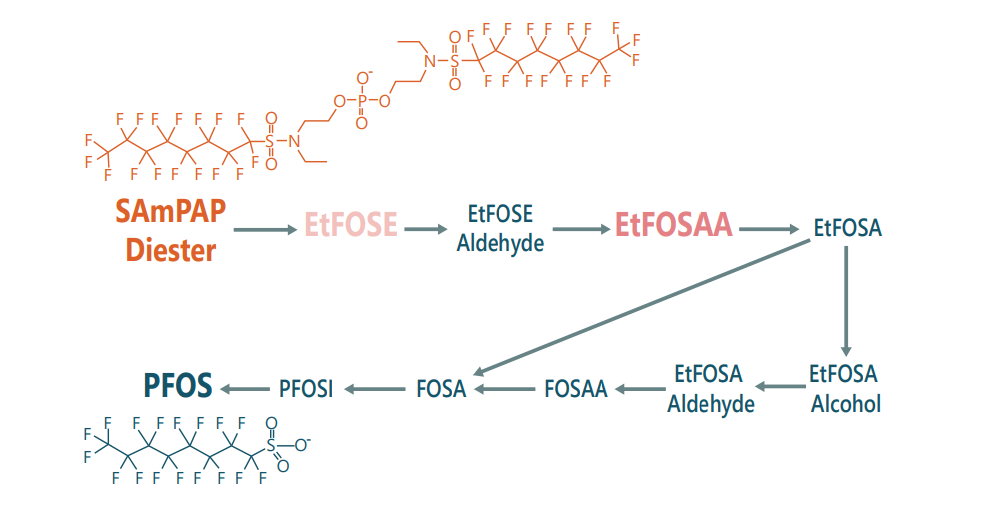
The exposure assessment for PFOS and PFOA is complicated not only by the presence of precursors but also by their transformation. SAmPAP is among the highest quantity PFOS precursors manufactured.
Because of these transformation pathways, the extent to which PFOS, PFOA, and other PFAS may pose risks in an aquatic environment depends on the fate and transport processes not only of these chemicals but also of their precursors. Understanding precursors and their chemical properties, fate, transport, and transformation in the environment is critical to setting effective water quality criteria, identifying PFAA sources, and reducing risks posed by PFAAs and their precursors.
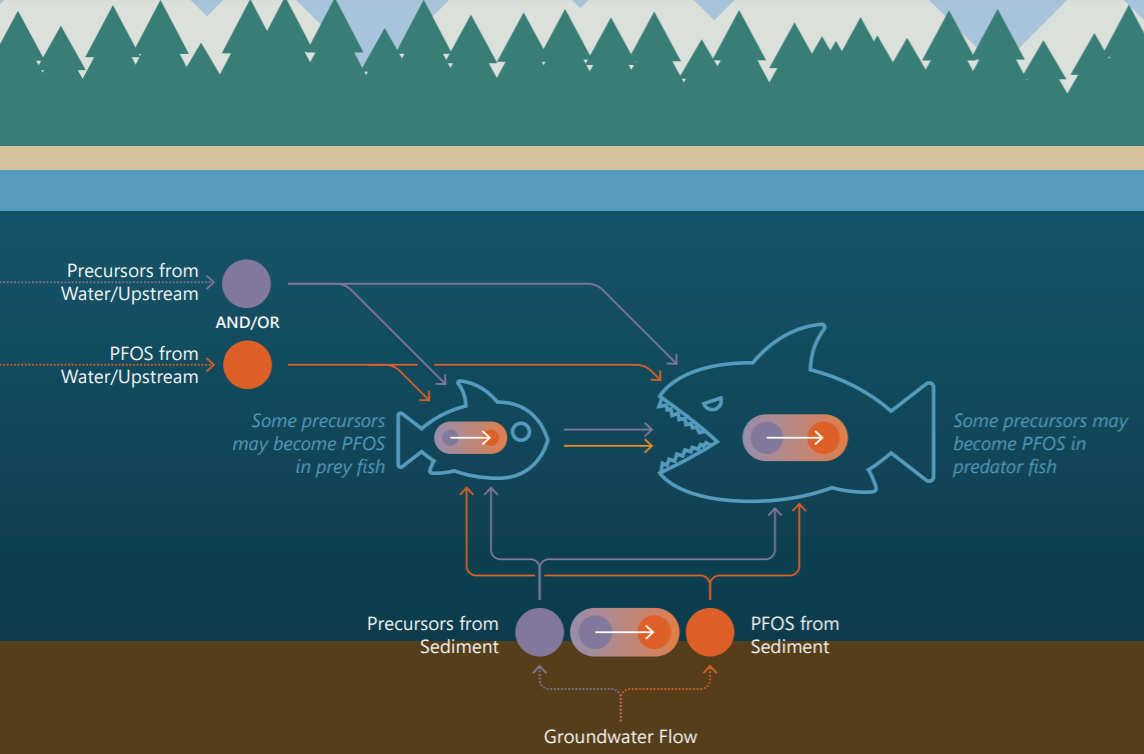
Assessing exposure and developing remedial strategies may depend on the relative concentrations and properties not only of PFOS but also of their precursors.
Circumstances Supporting Active Remediation of Aquatic Sediments
Unlike chemicals such as mercury and PCBs that typically drive sediment remediation, PFAAs are expected to be released relatively rapidly from sediments because of their weak affinity for particulate organic matter. Therefore, sediments are not typically expected to be a long-term source of PFAAs to the overlying water column; however, sediments may become a significant reservoir if precursors with higher sorption capacity continue to release PFAAs over time. Thus, remedial decision-making must consider the properties of PFAAs as well as their precursors.
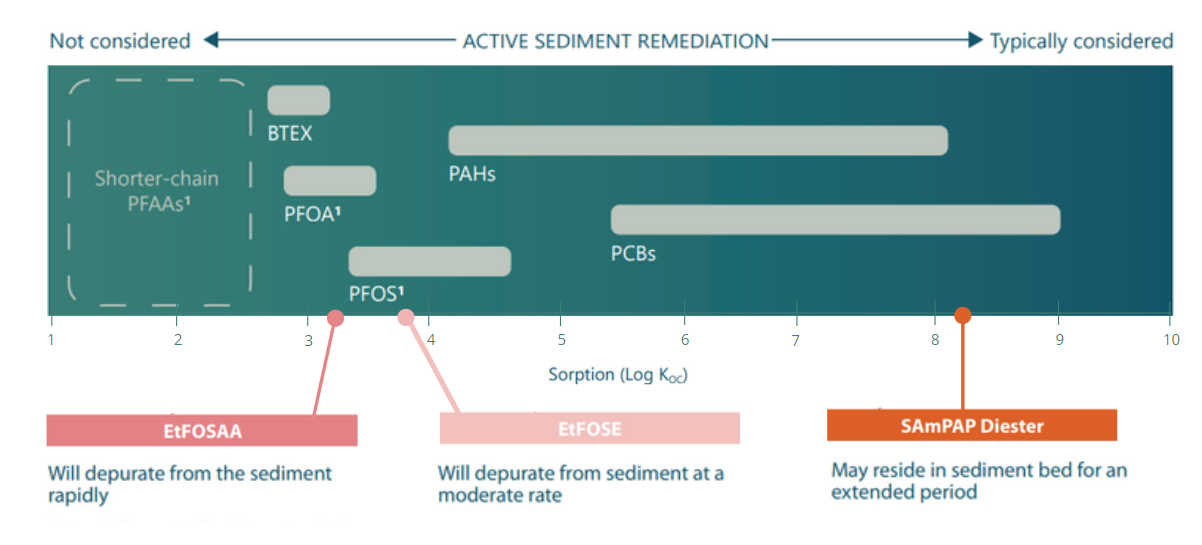
The chemical properties of PFAAs and their precursors influence the tendency of sediments to act as a long-term reservoir, and thereby influences remedial options . SAmPAP has been shown to reside in the sediment bed for an extended period.
The importance of aquatic sediments to PFAA exposure in the water column depends largely on two properties of the precursors: their transformation rates and the strength with which precursors bind to sediment organic matter. If transformation rates are relatively fast, PFAAs will be released rapidly from the sediment bed—but the precursors will also disappear rapidly due to that transformation. If transformation rates are very slow, the rate at which PFAAs are produced may not lead to significant exposure. But if a precursor exhibits strong sorption and intermediate transformation rates, PFAA exposure may continue for a significant length of time.
In addition to precursor transformation rates, characteristics of the sediment environment—including organic carbon content, particle mixing rate, groundwater advection rate, and diffusive exchange—control the release of PFAAs to the overlying water. Thus, the conditions that may create a long-term source of exposure to PFAA in the sediment bed and water column depend on a complex set of physical and chemical processes. Consideration of these processes is needed for remedial decision-making, both to effectively address the problem and to avoid unnecessary remediation that does not address the long-term source of the target chemical.
Sediment and Surface Water Model: Precursors Acting as a Long-Term Source of PFAS
To help bridge the gaps between risk assessment, PFOS bioaccumulation, and decision-making for contaminated sites, Anchor QEA is developing a fate and transport model of PFAS. Our objective is to support investigations into the sources of PFAS exposure, to provide general insights regarding PFAS fate and transport, and to inform remedial decision-making by providing information about PFAS characteristics and the site conditions under which PFAS may be a sediment management concern. Model simulations help evaluate how combinations of precursor transformation rates, partitioning, diffusion, and groundwater flux affect PFAA concentrations in the water column (and hence, potentially, remediation decisions).
Our team is developing a 3D hydrodynamic chemical fate and transport model of a sediment bed and overlying water column. We are using it first to simulate a typical riverine system. Such modeling will explore the conditions under which precursors may result in a significant sediment reservoir. Those conditions include the following:
- The precursor is relatively immobile and hence provides a long-term source of PFAAs.
- The transformation rate of precursor to PFAA is fast enough to result in significant PFAA concentration in the sediments and water column.
- The transformation rate is slow enough that the sediment constitutes a long-term source of PFAAs.
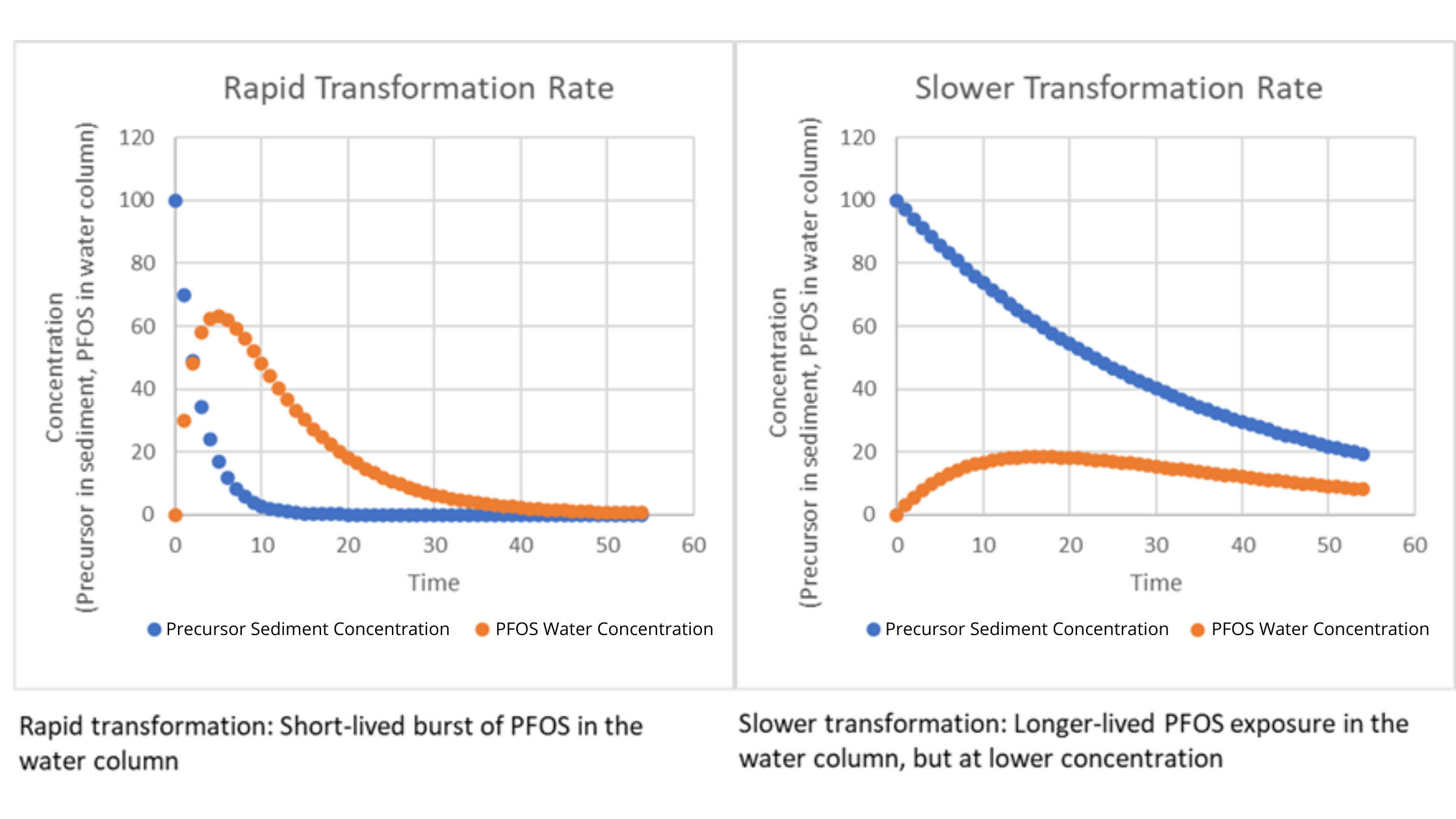
Time courses of precursor in sediment and PFOS in the water column in a river system. In this simple model, the precursor is depleted due to transformation. PFOS is created by the transformation process and diluted in the water column. This illustrates the interplay between transformation rates and other physical-chemical processes that affect the trajectory of PFOS to which the food web is exposed.
Preliminary results from the model indicate that precursors can potentially be a long-term source of PFOS, but only under specific conditions. Sediments where no precursors present are unlikely to be significant long-term reservoirs; in such cases, the sources of PFAA in the environment must be sought elsewhere (e.g., in groundwater, soil, or ongoing discharges). If precursors present in sediments have moderate to high sorption rates and moderate transformation rates, the sediments may be a significant reservoir and their remediation might be considered as part of an effort to reduce exposure. The overarching conclusion is that site investigations of PFAA contamination should include consideration of precursors.
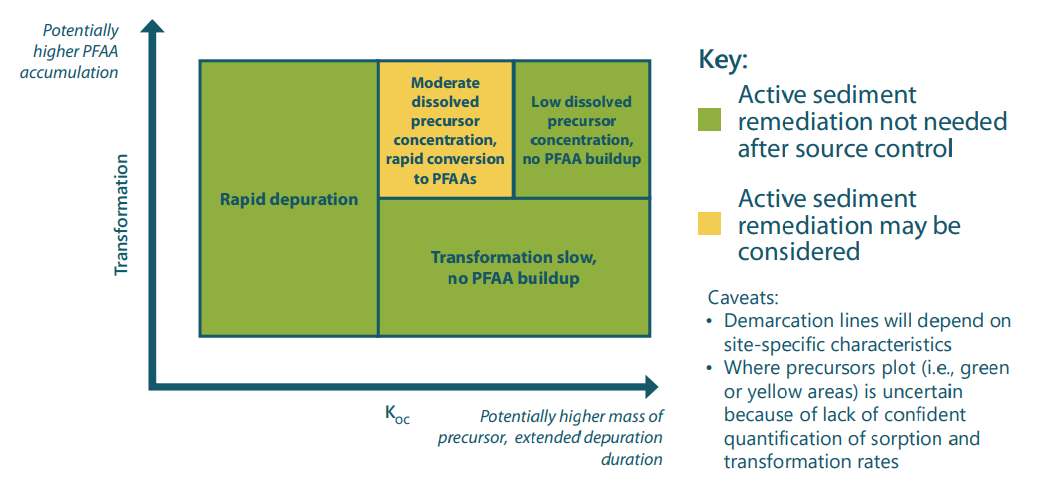
Conditions under which precursors may result in the need for active sediment remediation.
We are continuing to develop a suite of mechanistic models that includes PFAA precursor transformation, including the hydrodynamic chemical fate and transport model just described, a bioaccumulation model, and a groundwater fate and transport model, to better understand how PFAA precursors behave, transform, and move within the environment. The insights gained from these models will help project teams develop cost-effective management programs to successfully address PFAS contamination in the environment.
This is the third in a series of “Understanding PFAS in the Environment” articles. Future blogs in the series will provide more information on the groundwater model.
Authors

Elizabeth Lamoureux, ENV SP
Senior Managing Scientist
blamoureux@anchorqea.com |

David Glaser, PhD
Principal
dglaser@anchorqea.com |
Related Insights
Addressing PFAS in the Environment Part 2: Anchor QEA’s Bioaccumulation Model
April 29, 2021 – David Glaser and Dan Opdyke
Continue Reading »PFAS in the Environment Part 4: Anchor QEA’s Fate and Transport Groundwater Modeling
January 25, 2022 – Mike Gefell, Principal Scientist
Continue Reading »
